Dantek and mining industry
We make it possible to use the extractive element bit by bit
Micro nano-bubble in flotation and its application in mining industry
Today, micro-nanobubbles have various applications in flotation and mining industry. In general, flotation by using foam or froth floatation is a very effective method of particle separation in the mining industry. The mechanism of this method, like all flotation methods, is based on the difference in density and flotation of suspended particles, but there are some differences due to design limitations or the needs of the mining industry.
In this method, floating particles or particles in the fluid are separated from the fluid and concentrated using flotation process. The amount of product obtained from this method depends on the amount of mass made and the adhesion of the hydrophobic particles to the air bubbles. In general, the smaller the particles, the lower the probability of them hitting the bubbles and attaching to it, and the lower the probability of the particle getting stuck in a bubble mass.
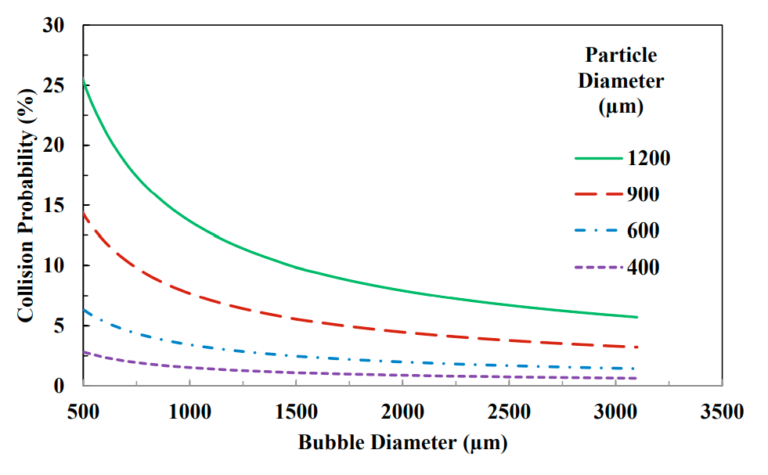
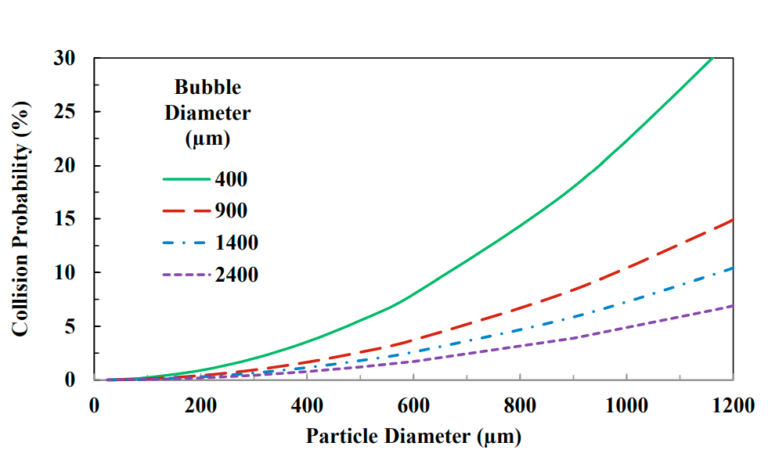
This is the main reason for the reduction in the flotation rate of fine particles in mineral concentration processes. Figures 1 and 2 show how reducing the bubble size can increase the probability of a particle colliding with the bubble, which ultimately leads to an increase in the particle separation rate and recovery rate.
Micro-nanobubbles and the definition of micro-nanobubbles in flotation
According to the classifications made and regarding to the size of the bubble, bubbles with a size of 200 nanometers to 10 micrometers are in the category of micro-nanobubbles. According to this classification, bubbles smaller than 200 nanometers are called nano-bubbles. The presence of these bubbles in the solid-liquid interface has been demonstrated by AFM atomic microscope.
Unlike macro-bubbles, micro-nanobubbles show very different properties in particle size distribution, mass transfer efficiency, common surface properties and chemical properties. These features have led to their increasing use in many fields of engineering, especially environmental pollution.
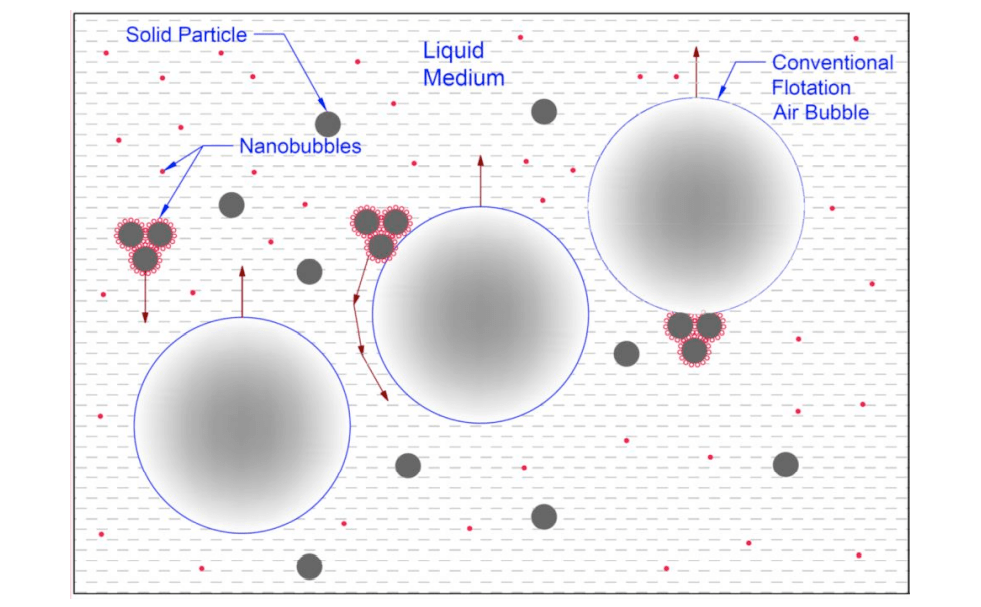
In the mining industry and in the flotation process, both bubble production ranges are required, and as will be explained below, nano-sized bubbles (at the nanoscale) will play an active role in trapping and capturing fine particles. In general, micro-nanobubbles in flotation can lead to increased recovery and extraction of minerals and fine particles, which means the extraction of materials that were previously out of reach due to difficulty in extraction.
Today, in addition to the use of bubbles, the use of nano-ozone is also increasingly used in the mining industry.
General characteristics of micro-nanobubbles and the basis of their production
Micro-nano-bubbles are very useful because of their characteristics. These characteristics mainly include, very small bubble size, large specific surface area, long durability time in water and fluid medium, negative zeta potential on the bubble surface, high mass transfer efficiency, and their ability to produce hydroxyl radicals. These properties of micro-nanobubbles are generally not found in any of the larger bubbles than micro-nanobubbles.
One of the most industrial methods of producing micro-nanobubbles is the use of two-phase vortex current and change in dissolved gas pressure. In this method, it dissolves in the fluid under conditions of air pressure or pressure of any other gas and dissolves in the fluid as supersaturation. Then the pressure is suddenly removed from the gas-liquid mixture, the gas suddenly comes out of the supersaturated state and expands and shows itself in the form of micro-scale bubbles.
Mechanism of separation of small particles by micro-nanobubbles in flotation
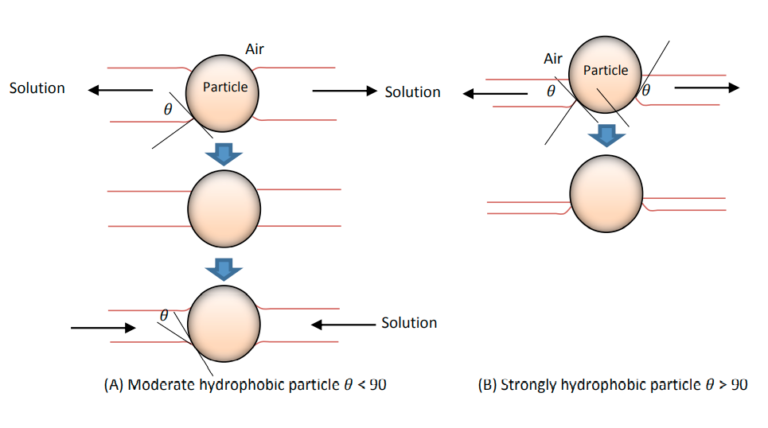
When micro- and nano-sized bubbles are used, images viewed using a scanning atomic microscope show that these bubbles adhere to very small particles and act as a capillary gas bridge to absorb hydrophobic particles to each other. The formation of this capillary bridge and the mechanism by which particles attach to each other are shown in Figure 3. The attachment of hydrophobic particles to the surfaces to which the nanobubbles adhere and form a coating for the particle causes a mass of particles to form.
This particle mass will definitely be larger than one particle, and this will eventually lead to more particles colliding with larger bubbles and better recovery of mineral particles. Therefore, very fine particles float and recover at a higher rate.
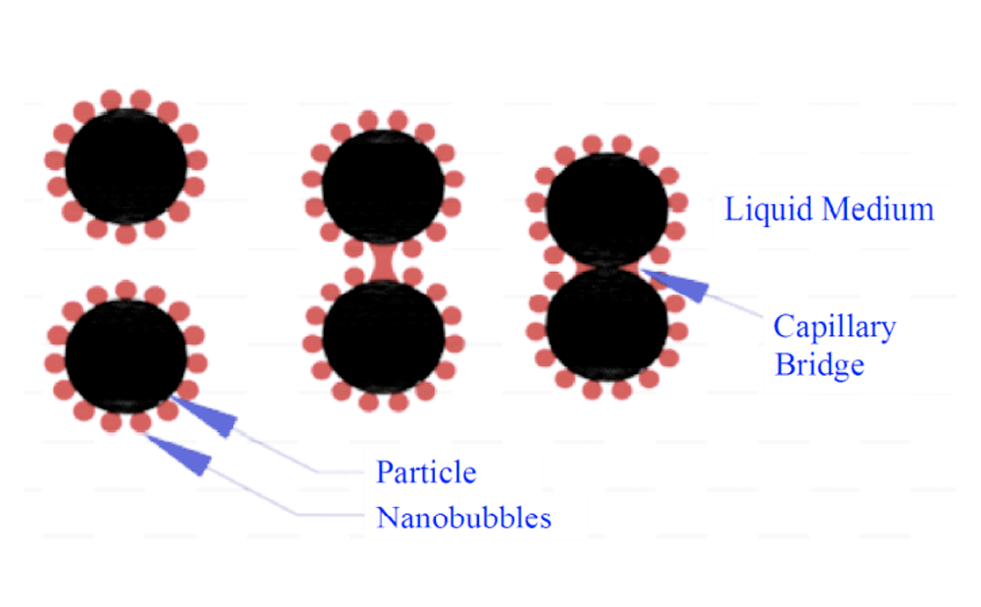
Mechanism of adhesion / separation of particles to micro-nanobubbles in flotation
After the particles collide with the bubbles, there is a possibility that the bubble will stick to the particle and separate. In the following, the possibility of the particles colliding with the micro-nanobubble in flotation will be investigated.
Mechanism of particles adhering to micro-nanobubbles in flotation
As the particles shrink, this time decreases and the chance of trapping the particles decreases. . One way to overcome this problem is to use water-friendly materials, in this case more particles become hydrophilic and the separation process is faster. Of course, this is associated with increased costs and consumption of surfactants. In this case, the best method is to use bubbles with small diameter and nanoscale bubbles.
Recently, research has been conducted on the effect of micro-nano-bubbles on flotation, which shows that nano-sized bubbles destroy the fluid layer near the mineral particles, thus allowing these particles to attach to the micro-sized bubbles. This principle is in fact the most basic principle in the flotation process.
In general, measurements made by micro-nano-bubbles in flotation have shown that the best way to form a wet layer and bond hydrophobic particles to each other is that the size of the nano-bubbles is equal to the size of the wetting film layer containing mineral particles. . If the size of the nanobubbles is less than the thickness of the wetting film, these micro-nanobubbles will play a small role in flotation and will have no effect on the rupture of this layer. Figure 4 shows the wetting film layer and nanobubbles smaller than this layer.
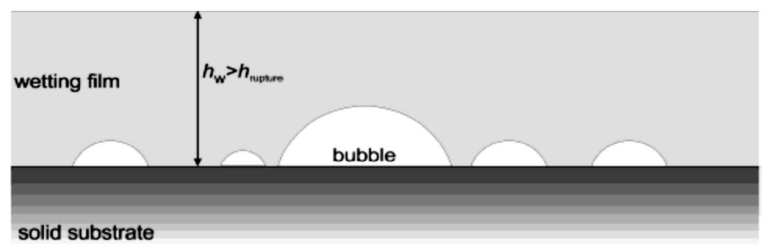
As the size of the wetting film layer and the nanobubbles gets close to each other, the surface force begins to show. Failure and rupture of the wetting film layer always occur between the largest bubble and the surface layer. This is due to the very thin water layer between the bubble and the wetting film surface; Therefore, the weakest point in the fluid layer is this point. This phenomenon is shown in Figure 5.
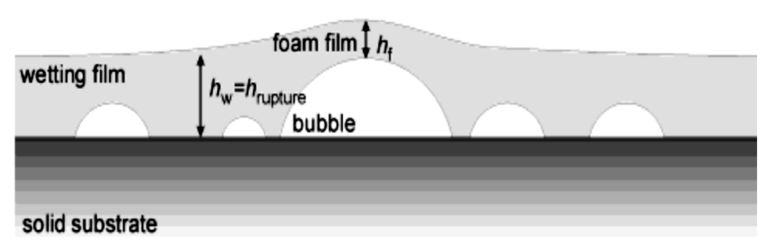
Possibility of particle separation from micro-nanobubbles in flotation
After the bubble hits the particle as well as sticking to each other, the probability that all of these particles and bubbles will remain connected to each other is still limited, and some of the bubbles usually separate from the particles and the particles enter the pulp phase. The probability of this separation is due to factors such as the energy of the bubble-particle collision, the energy required for the particle bubble separation, and the work of the bubble adhesion.
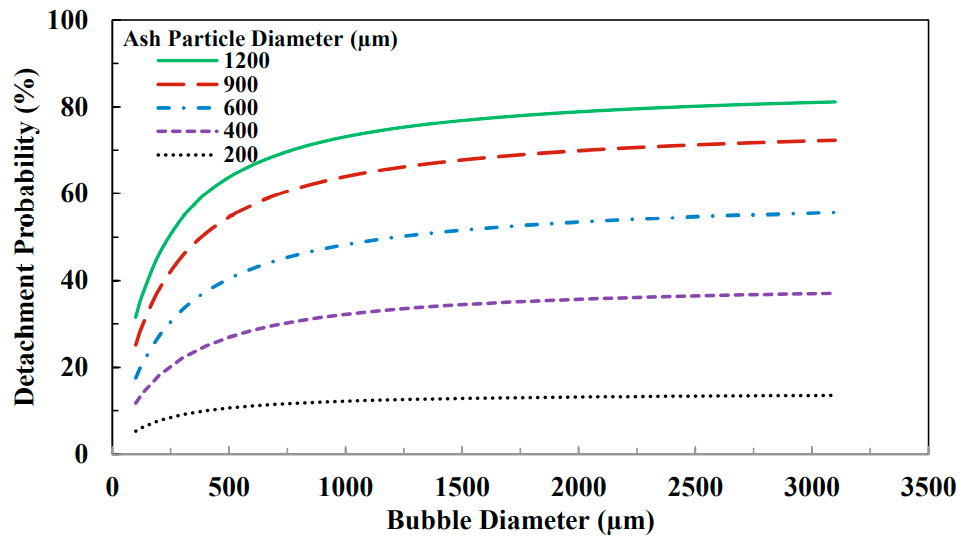
Separation generally occurs when the separation force is greater than the maximum adhesion force. The probability of bubble particle separation increases with increasing bubble diameter. Therefore, larger and heavier hydrophobic particles are more likely to separate from larger bubbles, and the smaller the bubble, the higher the recovery rate, even for large particles. Figure 6 shows this case.
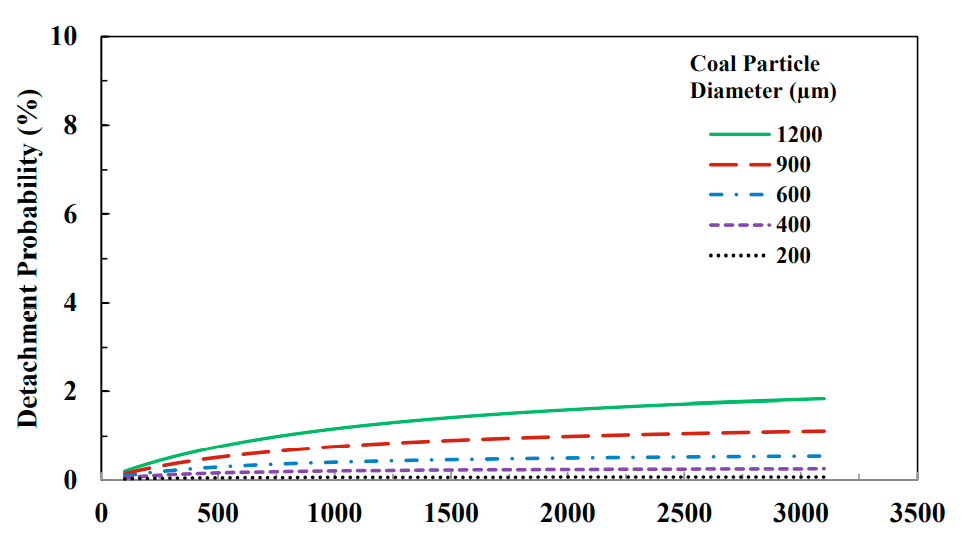
Particle size range in flotation systems
One of the most important factors in determining the flotation efficiency of particles in the mining industry goes back to the particle size. If the suspended particles are smaller than a certain range, the probability of collision decreases, and if these particles are larger than a certain range, the probability of their adhesion decreases. However, if the nanobubbles are placed on smaller particles and completely surround them, they will bind these particles together and thus increase the recovery coefficient. This is due to the increased probability of particles colliding with each other.
In addition, nano-bubbles increase the hydrophilicity of the particle, which causes the bubble and the particle to adhere better and separate later. The use of micro-nanobubbles in flotation increases the size range of separable particles in the separation process, resulting in an increase in the amount and rate of recovery. This is due to the relative increase in impact rate, adhesion strength and reduced separation probability.
Some research conducted until now has shown that the use of micro-nanobubbles in flotation can reduce the separation range of fine particles to particles a few microns in size (even less than one micron) and separate larger particles by about 1 to 2 millimeter. This can also increase the use of flotation systems in the mining industry (1).
Many reports today show that the recovery rate in flotation increases when nanobubbles are placed next to normal-sized bubbles, and there are two general mechanisms for this.
- The formation of a nanobubble layer on hydrophobic particles that causes the formation of a mass, and this is due to the existence of the bubble bridge and its related mechanism. This mass formation eventually leads to an increase in the collision rate.
- •Particles coated with nano-bubbles have a greater potential to adhere to normal-sized bubbles, thus increasing their separation rate.
Foam phase in flotation (foam produced by micro nano bubbles in flotation)
In the flotation process, the hydrophobic particles are trapped using air bubbles and brought to the surface of the slurry-like layer in the foam area. This foam overflows when it comes to the surface and exits the flotation chamber. Unlike slurry-like phase, floor-like phase shows completely different properties and behavior. When the bubble rises and the accompanied water remains, the foam starts to grow as it rises.
In some cases, the top layer may be completely dry if the bottom layer is still wet. When washing water is added to the system, water places on the foam and soaks it. Therefore, the foam layer is the most important part of the concentration process and determines the final grade and recovery rate.
The bubbles that form in this area in the shape of a sphere are very fragile and unstable. The stability of these bubbles, in addition to the concentration of chemicals, also depends on the nanobubbles inside the foam layer. Maximum stability is achieved when the foam layer contains particles with moderate hydrophobicity that can form stable air bridges in the foam-like layer. If the hydrophobicity of the particles is very high or there are particles with asymmetric shapes, it causes the foam layer to be unstable and the reason is the bursting of bubbles and the destruction of the air bridge between them. A picture of this phenomenon is given in Figure 7.